Abstract
Introduction
The outcome of conventional allogeneic hematopoietic cell transplantation (HCT) for the treatment of patients with refractory myeloid malignancies remains poor. Thiotepa-based sequential conditioning has shown promising results for various refractory hematological malignancies (Duléry R et al. Biol Blood Marrow Transplant. 2018; 24(5):1013-1021). However, no study has evaluated the outcome of this sequential approach specifically in refractory myeloid malignancies. The optimal dose of chemotherapeutic agents used in the conditioning regimen and the feasibility of this sequential approach in patients aged 60 years and above also need to be assessed.
Methods
All consecutive patients with refractory myeloid malignancy who underwent allogeneic HCT with a thiotepa-based sequential conditioning regimen in Saint Antoine hospital between April 2013 and October 2020 were included. The sequential approach consisted of a broad spectrum cytoreduction with thiotepa, etoposide, and cyclophosphamide (TEC) from day -15 to -10. Then, after a 3-day rest, a reduced-intensity conditioning (RIC) regimen was administered with fludarabine 150 mg/m 2, intravenous busulfan 6.4 mg/kg and thymoglobulin 5 mg/kg from day -6 to -2. From 2013 to 2018, doses of thiotepa (10 mg/kg), etoposide (400 mg/m 2) and/or cyclophosphamide (1600 mg/m 2) could be reduced in patients older than 60 years or with comorbidities. Since September 2018, the use of a reduced TEC-RIC regimen (thiotepa at 5 mg/kg, etoposide 300 mg/m 2 and cyclophosphamide 1200 mg/m 2 from day -13 to -10) became the new standard of care for all patients. After transplant, patients could receive a hypomethylating agent or targeted therapy to prevent relapse. Prophylactic donor lymphocyte infusions were given to enhance the graft-versus-leukemia effect, in the absence of contraindication. Graft-versus-host disease (GVHD) prophylaxis was cyclosporine A and mycophenolate mofetil for all patients. Post-transplant cyclophosphamide was added in the case of haploidentical HCT.
Results
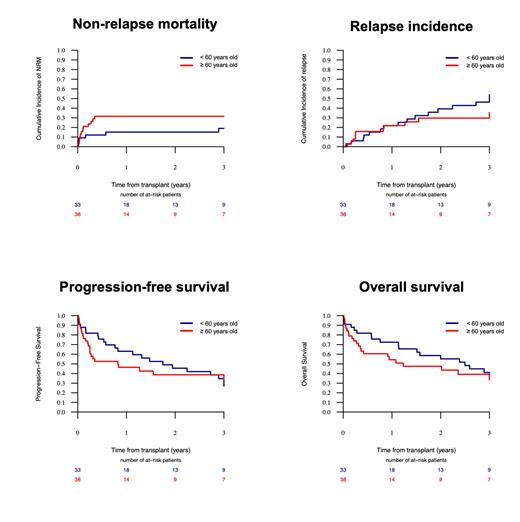
Seventy-one patients (median age 61 years, range 15-76) were included. Diagnoses comprised acute myeloblastic leukemia (n=65) and myelodysplastic syndrome/chronic myelomonocytic leukemia (n=6). Donors were haploidentical (n=36), matched related (n=13), unrelated (n=21) and umbilical cord blood (n=1). A reduced TEC-RIC was administrated to 43 (61%) patients, a full dose TEC-RIC to 18 (25%), and a TEC-RIC with a dose reduction only for thiotepa to 10 (14%). With a median follow-up of 45.4 months (95% CI: 36.7-63.6), the 2-year overall survival (OS), progression-free survival (PFS), and non-relapse mortality (NRM) were 51%, 41%, and 24%, respectively. Patients receiving a reduced TEC-RIC had a lower cumulative incidence of acute and chronic GVHD (acute grade II-IV GVHD: 14%, acute grade III-IV GVHD: 0%, chronic GVHD: 19%) than the other patients (acute grade II-IV GVHD: 29%, acute grade III-IV GVHD: 18%, chronic GVHD: 29%). The outcomes were not significantly different according to the TEC dose in terms of relapse incidence, OS, and PFS, although the NRM was lower with the reduced TEC-RIC (21% versus 28%, p=0.72). Most notably, patients aged 60 years and above had a 2-year OS and PFS of 48% and 39%, respectively, with no significant difference compared to patients younger than 60 years (Figure).
Conclusion
Our findings endorse the feasibility and efficacy of a thiotepa-based sequential approach for refractory myeloid malignancies undergoing HCT. The dose reduction did not compromise disease control and expanded the transplant option to older patients, ineligible for a higher dose regimen. Thus, a reduced TEC-RIC sequential regimen can be proposed for selected patients older than 60 years and allows promising outcomes for the treatment of refractory myeloid malignancies.
Duléry: Gilead: Other: travel support and meeting fees; Takeda: Consultancy; Novartis: Honoraria. Malard: Therakos/Mallinckrodt: Honoraria; Biocodex: Honoraria; Astellas: Honoraria; JAZZ pharmaceuticals: Honoraria; Sanofi: Honoraria; Janssen: Honoraria. Legrand: Servier: Consultancy. Fenaux: Abbvie: Honoraria, Research Funding; JAZZ: Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Labopin: Jazz Pharmaceuticals: Honoraria. Mohty: Sanofi: Honoraria, Research Funding; Pfizer: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Jazz: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria; Celgene: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Astellas: Honoraria; Amgen: Honoraria; Adaptive Biotechnologies: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal